
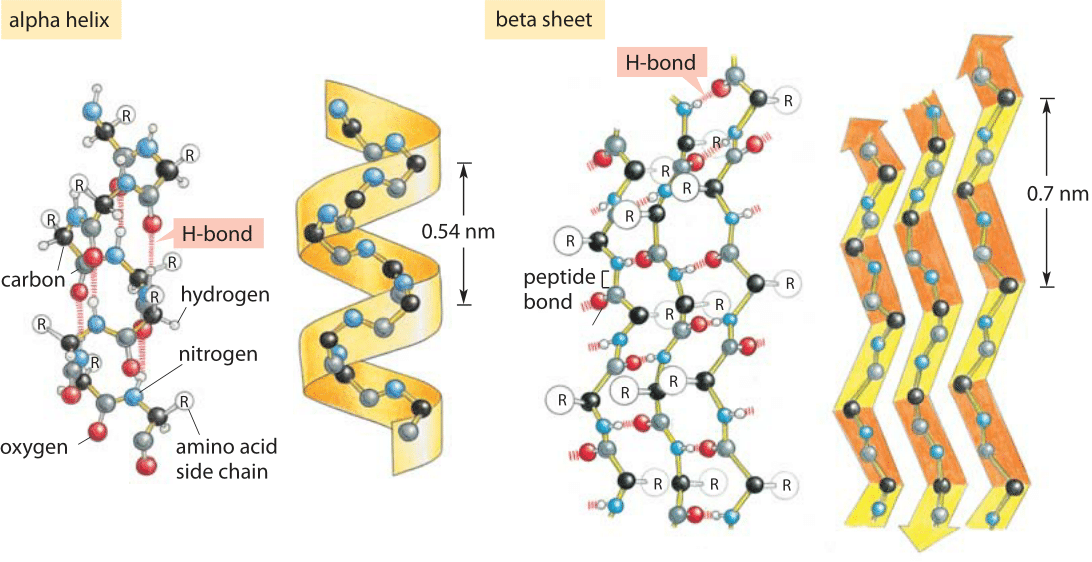
On the left is a CPK representation (without sidechains) looking down the helical axis from the C-terminus showing the four non hydrogen-bonded carbonyl oxygens. A C-terminal Gly-based capping motif named for its discoverer (Schellman, 1980). In each of these motifs, the C' residue must adopt a left-handed helical conformation which is why Gly is favored although residues with beta carbons are found. The other motif ( alpha-L) contains one hydrogen bond connecting the backbone amide of C' to the backbone carbonyl oxygen of C3. One motif ( Schellman motif) contains two "capping" hydrogen bonds, one connecting the backbone amide of C'' to the backbone carbonyl oxygen of C3 and he other connecting the backbone amide of C' to the backbone carbonyl oxygen of C2 ( Figure 4 ). Helices terminating in Gly can be classified into to primary motifs based on their hydrogen bonding patterns. The "capping box" hydrogen bonds are shown as black arrows, whereas the helical hydrogen bonds are shown as yellow dashed lines.Īpproximately one-third of all alpha helices contain a Gly-based capping motif at the C-terminus. The four non hydrogen-bonded amide nitrogens are colored blue and the side chains of Ncap and N3 are also shown. On the right, the helix is again shown this time using stick representation following a 90 degree rotation. On the left is a CPK representation (without sidechains) looking down the helical axis from the N-terminus showing the four non hydrogen-bonded amide nitrogens. A typical "capping box" from cytochrome 351. Here the hydroxyl oxygen forms a standard Ncap hydrogen bond with the amide of N3 and the side chain carbonyl oxygen of N3 forms a hydrogen bond with the amide of Ncap thus satisfying two of the four non hydrogen-bonded helix N-terminal amides.įigure 3. A special form of Ncap utilizing a reciprocal hydrogen bonding motif is called the " capping box" and is shown in Figure 3. Typical Ncap interactions involve a hydrogen bond from the side chain hydroxyl oxygen of Ser and Thr or side chain carbonyl oxygen of Asn with the backbone amide nitrogen of N3. The Gly in the Schellman motif is called Ccap and C', respectively. Note: there appears to be a nomenclature inconsistency between Richardson & Richardson, 1988 and Aurora et al., 1994. Table II: Selected residues with statistically significant positional preferences (colored regions data taken from Richardson & Richardson, 1988). Strong amino acid preferences are shown in the table below: In their survey of 13 proteins containing 54 alpha helices, 48% of the Ncap-N1-N2-N3- residues and 35% of the C3-C2-C1-Ccap- residues were satisfied by local side chain or backbone hydrogen bonds (as nearly all potential hydrogen bonding groups in a protein are satisfied, the remaining are satisfied by amino acids brought into position by the folding of the protein or by solvent molecules). The eight terminal residues lacking hydrogen bond partners are then Ncap-N3 and Ccap-C3. Where N1 through C1 are residues which contain alpha-helical phi, psi angles and the primed residues belong to the residues outside the helix on either end. The proposed nomenclature uses the following definition: Helix boundary residues (the first and last helical residues) are called Ncap and Ccap at the N- and C-terminus, respectively (Richardson & Richardson, 1988). Furthermore, these residue positions were found to contain differentiated amino acid preferences. It has been shown that hydrogen bond partners for these otherwise unsatisfied first four amide protons and last four carbonyl oxygens are often provided by polar groups that flank the helix termini (Presta & Rose, 1988 Richardson & Richardson, 1988). The N- and C-terminal ends of an isolated helix contain four NH donors and four CO acceptors each, respectively due to edge effects ( Figure 2 ). 3.1.4 Selected topics Ī 12 residue alpha helix will contain only 8 hydrogen bonds, despite the 12 backbone NH (donors) and 12 backbone CO (acceptors).


 0 kommentar(er)
0 kommentar(er)
